Alzheimer's Disease

Alzheimer’s disease (AD) is a multifactorial chronic neurodegenerative disease characterized by cognitive impairments and synaptic failure. Concomitant with the rapid growth of the aging population, incidence of Alzheimer’s disease is rising worldwide, becoming a global threat to public health and potentially a new epidemic for the 21st century. Unfortunately, no interventions have been discovered to either slow the progress of AD or cure the disease, and recent clinical trials have not succeeded in identifying disease-modifying strategies. The research in our lab focuses on understanding of the molecular signaling mechanisms underlying pathophysiology of AD, thus providing insights into identification of novel therapeutic avenues and/or diagnostic biomarkers for AD. The cartoon figure shown here illustrates several signaling cascades that are dysregulated in AD including mammalian target of rapamycin complex 1 (mTORC1), mRNA translational factor eukaryotic elongation factor 2 (eEF2) and its kinase eEF2K, AMP-activated protein kinase (AMPK). Using multiple approaches including those in pathology, cell biology, synaptic electrophysiology, confocal imaging, mouse genetics, and behavioral tests, we aim to determine the roles of these signaling pathways (when they go awry) in AD etiology.
Down syndrome (DS) is one of the most common forms of intellectual disability due to a trisomic repeat of chromosome 21. Currently there are no pharmacological treatments that improve cognitive deficits in those individuals. Of note, several genes encoded on chromosome 21 that are overexpressed in DS are also implicated in Alzheimer's disease. Consistently, most people with DS develop typical AD brain pathology by age 40, and show symptoms of Alzheimer's disease such as dementia in their 50s or 60s.
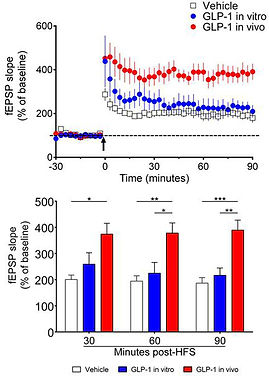
Glucagon-like paptide 1 (9-36) or GLP-1(9-36) is thethe cleavage product of the insulinotropic hormone GLP-1 (7-36). Originally GLP-1 (9-36) was considered as a "bioinactive metabolite mainly because of its lack of insulinotropic activity and low affinity for the GLP-1 receptors. However, mounting evidence demonstrates that GLP-1 (9-36) possesses important biological functions. For example, we previously shown that GLP-1 (9-36) treatment improves memory and synaptic plasticity impairments in AD model mice. Most recently, we have shown that systemic administration of GLP-1 (9-36) enhances hippocampal long-term synaptic plasticity (Day, 2017), as shown in the left figure.
Currently we are investigating whether GLP-1 (9-36) treatment is able to alleviate cogntive deficits and synaptic failure in a mouse model of DS.
Down Syndrome
Research Funding
NIH/NIA K99/R00 AG044469 (Ma)
NIH/NIA R01 AG055581 (Ma)
NIH/NIA R01 AG056622 (Ma)
NIH/NIA F31AG054113 (Beckelman)
NIH/NIA F31AG055264 (Zimmermann)
BrightFocus Foundation
A2017457S (Ma)
Alzheimer's Association
NIRG-15-362799 (Ma)
Wake Forest School of Medicine
ADCC pilot grant, CTSI pilot grant, Start-up fund



